With prayer, Gods loving guidance and support from family and friends.....
An update on my 25 year old son who has EMBRYONAL CARCINOMA. I have placed a detailed description of what this is below the video.
Justin's case is not exactly typical, as his cancer spread to his lymph system and other areas. The lymph system in his abdomen is fully affected, a node in his stomach, liver as well as larger tumors in several locations in both his lungs. They considered him a stage 4 cancer patient. He has had 3 rounds of chemo, which has caused problems with hearing loss and heart issues. But the tumors have shrunk, he maybe considered a stage 3 cancer patient. After this fourth round of chemo he will rest, get stronger and then go to Dana Farber in Boston for surgery....please continue to pray.
Justin has rested for the past week and a half...we have all rested~there was no chemo!!!! He did have doctors appointments and so far everything is stable. He is responding well to the heart meds he now has to take and is still battling nausea from time to time, (but not like chemo days). He will start chemo......his LAST round, (GOD WILLING) next week. Courtney his girl friend, will bring him to chemo and stay until noon-ish; then Tim will will relieve her and drive him home, (to our home) so he can rest and eat until evening (when Courtney gets to their home.... from work). It is the same routine we have maintained through out this whole ordeal.
This young man is named LUCAS PEPKE he is the main cantor for the EWTN daily Mass.
EMBRYONAL CARCINOMA
Embryonal carcinoma is a germ cell tumor characterized by primitive epithelial cells with marked pleomorphism and various histologic patterns. It may present in pure form but often is part of a mixed germ cell tumor.
Embryonal carcinoma is one of the most common germ cell tumors. The peak incidence occurs in persons aged 20-30 years. It is extremely rare in infants. About 3-10% of pure germ cell tumors are embryonal carcinomas, and embryonal carcinomas are present in more than 80% of mixed germ cell tumors. [1, 2]
Genetic and environmental factors play a role in the development of germ cell tumors, [3, 4] but the importance of specific factors is unclear. [5] The most accepted theory on the development of germ cell tumors involves an initiating event that causes fetal gonocytes to undergo abnormal cell division.
As with other germ cell tumors, with the exception of spermatocytic seminoma, embryonal carcinomas are believed to originate from intratubular malignant germ cells. Germ cell tumors show many features of normal embryogenesis, such as pluripotency, as governed by the expression of OCT3/4 (see Immunohistochemistry). [6] In fact, methylation studies have shown that epigenetic phenomena may play an important role in the genesis of germ cell neoplasias. [7, 6, 8, 9]
In the past, intratubular malignant germ cells were referred to as carcinoma in situ, [10] but, in the current, accepted nomenclature, the term intratubular germ cell neoplasia, unclassified (IGCNU), is used. [5] In the vast majority of cases, IGCNU occurs adjacent to invasive germ cell tumors, including embryonal carcinomas. [11, 12] In a series of testicular biopsies of patients with cryptorchidism, the rate of IGCNU was increased, and it is known that, overall, 10% of germ cells tumors are associated with cryptorchidism. [13, 14, 15]
Embryonal carcinomas are located in the testicular parenchyma. These tumors are usually smaller than seminomas. They do not replace the entire testis but have a propensity to invade paratesticular tissue (through the tunica albuginea), rete testis, and epididymis. [16] Vascular invasion is common.
Embryonal carcinomas are aggressive germ cell tumors. The peak incidence occurs in persons of about age 30 years. About 10-40% of patients (including those with pure and mixed forms) present with metastasis; the more common sites of metastasis are the retroperitoneum, lung, and liver. Initial evaluation includes testicular ultrasonography, but more advanced imaging techniques are used preoperatively and postoperatively to assess the status of the local disease and lymph nodes and to assess for the presence of distant metastasis. Because of the wide use of testicular ultrasonography, small, nonpalpable tumors are being reported more often than in the past. [17]
Although serum alpha fetoprotein (AFP) has been reported in association with embryonal carcinomas, most of those cases were probably misclassified as such and in truth represented mixed cases of embryonal carcinomas and yolk sac tumors. Association of embryonal carcinomas with syncytiotrophoblastic giant cells may cause increases in serum human chorionic gonadotropin (hCG) levels. A search for those elements may be clinically important in any given case. Levels of other serum markers, such as placental alkaline phosphatase (PLAP) and lactic acid dehydrogenase (LDH), may be elevated.
Embryonal carcinomas usually present as ill-defined masses. The cut surface may be smooth or lobulated. Foci of hemorrhage and necrosis are common. The average size is about 3-4cm in most series. Invasion into paratesticular tissue may be detected grossly in about 20% of the patients. It is difficult, if not impossible, to distinguish pure embryonal carcinomas from mixed germ cell tumors grossly; however, teratomatous elements or cystic changes are present in mixed tumors.
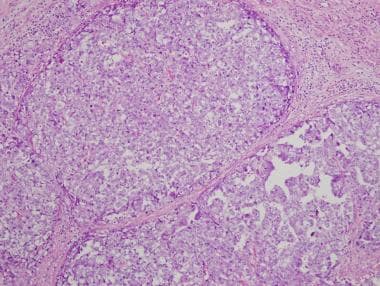
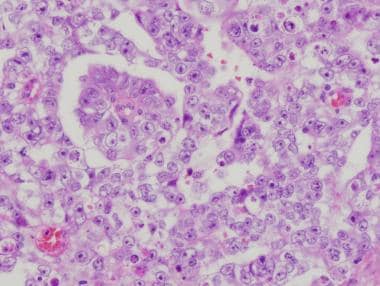
There are 3 main histologic patterns of embryonal carcinomas—solid (see the first image below); tubular, or glandlike (see the second image below); and papillary (see the third and fourth images below). Any given tumor may show a combination of these patterns. These different patterns, as well as cytologic features, help in distinguishing embryonal carcinomas from seminomas (see the fifth image below).
 Low-magnification view of embryonal carcinoma showing diffuse sheets of pleomorphic cells with tumor necrosis.
Low-magnification view of embryonal carcinoma showing diffuse sheets of pleomorphic cells with tumor necrosis.
 Tubular (glandlike) pattern of embryonal carcinoma. Note the long tubules and glandlike lumina surrounded by pleomorphic cells. The latter are either cuboidal, seen lining the lumina, or round to ovoid, seen in the solid areas.
Tubular (glandlike) pattern of embryonal carcinoma. Note the long tubules and glandlike lumina surrounded by pleomorphic cells. The latter are either cuboidal, seen lining the lumina, or round to ovoid, seen in the solid areas.
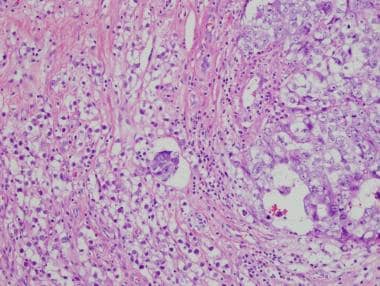 Mixed germ cell tumor. Visible on the right side of the image is embryonal carcinoma, characterized by pleomorphic cells with glassy nuclei and prominent nuclei. Cellular borders are indistinct. By comparison, seminoma is visible on the left side of the image. In seminoma, the cells are less pleomorphic and have conspicuous membranes.
Mixed germ cell tumor. Visible on the right side of the image is embryonal carcinoma, characterized by pleomorphic cells with glassy nuclei and prominent nuclei. Cellular borders are indistinct. By comparison, seminoma is visible on the left side of the image. In seminoma, the cells are less pleomorphic and have conspicuous membranes.
Diffuse sheets of anaplastic cells characterize the solid pattern, and one may commonly find punctuate foci of necrosis.
The tubular pattern may be prominent and cause confusion with yolk sac tumors, but cytologic features readily distinguish these neoplasms. The cells of embryonal carcinoma in the tubular pattern may have a columnar appearance, lining the glandlike lumina.
There are no prognostic differences associated with the histologic patterns; the type of pattern should not be included in the surgical pathology report unless as a comment regarding the differential diagnoses.
The necrosis seen in embryonal carcinomas is usually of the coagulative type, with dense eosinophilic debris and cellular debris. Mitoses are frequent. In some cases, there is a distinct spindle cell stroma around tumor nests. The recognition of this feature is important in distinguishing embryonal carcinoma from the stroma of teratoma, especially in cases of pure forms of embryonal carcinoma.
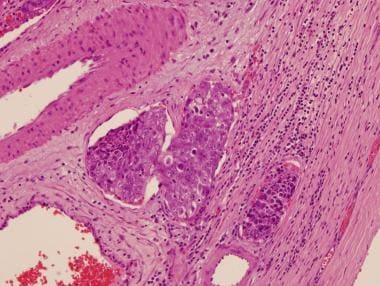
Intratubular embryonal carcinoma is commonly associated with embryonal carcinoma (see the image below). Intratubular embryonal carcinoma has a unique histologic appearance of densely eosinophilic necrotic debris involving native seminiferous tubules; it is surrounded by typical embryonal carcinoma cells.
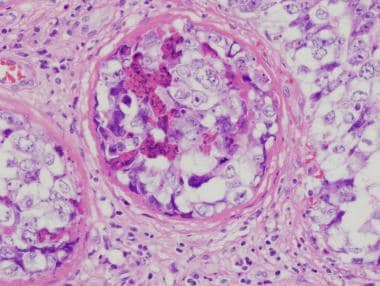 Intratubular embryonal carcinoma. Dense, eosinophilic, necrotic debris in association with embryonal carcinoma cells is filling the seminiferous tubule.
Intratubular embryonal carcinoma. Dense, eosinophilic, necrotic debris in association with embryonal carcinoma cells is filling the seminiferous tubule.
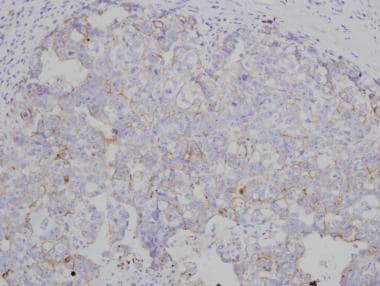
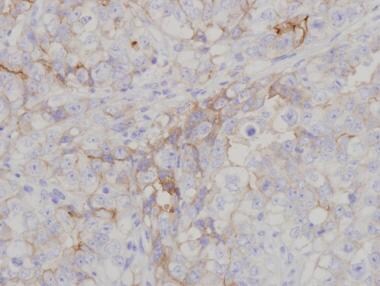
Intratubular embryonal carcinoma has been found to be associated with invasive tumor in approximately 7% of cases. [18] Vascular invasion is common; it affects prognosis and should be carefully excluded. Immunohistochemistry should be used in difficult cases (see the images below). A study by Gilbert et al reported that chemokine CXCL12 expression and percentage embryonal carcinoma both stratify patients' relapse risk over and above vascular invasion alone. The authors added that these results can improve the stratification of patients and identify high-risk cases to be considered for adjuvant therapy. [19]
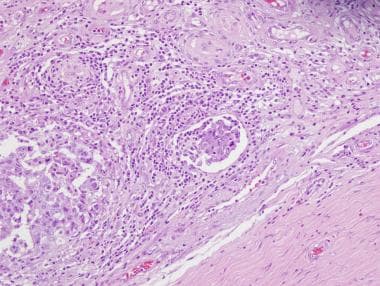 Low-magnification image of embryonal carcinoma showing a cluster of cells (center of the field) with retraction and empty space, which suggests invasion of the vascular space.
Low-magnification image of embryonal carcinoma showing a cluster of cells (center of the field) with retraction and empty space, which suggests invasion of the vascular space.
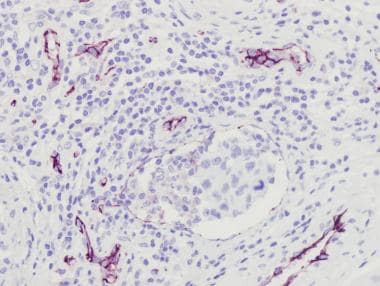 Same area seen in the image above after immunohistochemistry staining with CD31. Endothelial lining is apparent around tumor cells, confirming small-vessel invasion.
Same area seen in the image above after immunohistochemistry staining with CD31. Endothelial lining is apparent around tumor cells, confirming small-vessel invasion.
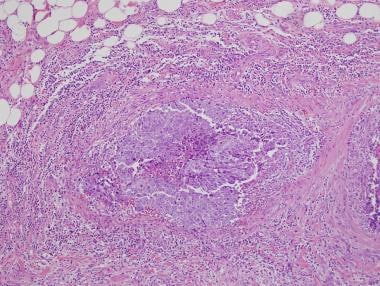
Extension into paratesticular tissue should be documented if present; it represents a change in pathologic stage (see the first image below). In difficult cases in which the differential diagnosis includes metastatic carcinomas, association with intratubular germ cell neoplasia supports a diagnosis of germ cell tumor (see the second image below).
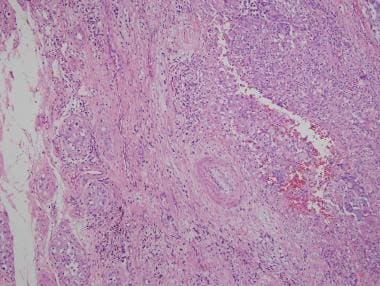 Embryonal carcinoma (right) in association with intratubular germ cell neoplasia on surrounding testis (left).
Embryonal carcinoma (right) in association with intratubular germ cell neoplasia on surrounding testis (left).
The histology of embryonal carcinomas is easily recognizable and is distinct from those of other common germ cell neoplasms, such as seminoma, yolk sac tumor, and choriocarcinoma. As stated above, immunohistochemistry may aid in the differentiation in difficult cases. Pleomorphic seminomas (so-called “anaplastic” seminomas or seminomas with high mitotic rate) may be confused with embryonal carcinoma. In older patients, large cell lymphoma and metastatic disease should be included in the differential diagnosis.
Embryonal carcinomas are typically positive for placental alkaline phosphatase (PLAP), c-kit (CD117), keratins (8, 18, 19), and CD30. In addition, embryonal carcinomas test positive for other markers now being used in the diagnosis, including NANOG, SOX2 (sex-determining region Y [SRY] – box 2), and OCT3/4. Seminomas are negative for CD30 and SOX2—a fact that should help in the differential diagnosis. AFP is only rarely positive in scattered embryonal carcinoma cells and usually distinguishes yolk sac areas. Markers used in the diagnosis of embryonal carcinoma are demonstrated in the images below. [20,21, 22]
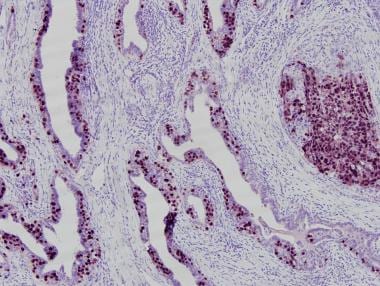 OCT3/4 immunohistochemistry marking embryonal carcinoma (solid area on right) and involvement of rete testis by embryonal carcinoma cells (left).
OCT3/4 immunohistochemistry marking embryonal carcinoma (solid area on right) and involvement of rete testis by embryonal carcinoma cells (left).
A study by Stoop et al found the direct enzymatic alkaline phosphatase reactivity (dAP) test to be an effective and easily used means of diagnosing embryonal carcinoma, as well as IGCNU and seminoma, intraoperatively, in testis-sparing surgery. Using 4093 snap-frozen samples for dAP testing, the investigators found that the results of the 15-minute dAP test compared favorably with those from hematoxylin and eosin staining and immunohistochemical detection of OCT3/4, AFP, hCG, and cytokeratin on paraffin-embedded – tissue slides. [23]
The cytogenetic abnormalities seen in embryonal carcinomas are similar to those of the other germ cell tumors (but not infantile yolk sac tumors, teratomas, and spermatocytic seminomas, which have a different genetic profile and clinical presentation). The most accepted theory on the development of germ cell tumors involves an initiating event that causes fetal gonocytes to undergo abnormal cell division. This process is accompanied by the formation of isochromosome 12p, or i(12p). The most common genetic abnormality found in germ cell neoplasms, i(12p) occurs in all histologic types in primary and metastatic sites. [5, 24]
The gene on i(12p) that may be involved in the carcinogenesis of germ cell tumors has not been identified. The i(12p) abnormality may be detected using commercially available probes and has great potential in clinicopathologic research. Currently, however, it does not have important applications in the clinical setting.
The pathologic staging of testicular germ cell tumors is based on 2 main features: (1) extension of the tumor outside the testicular parenchyma and (2) vascular invasion. The TNM classification of these tumors includes the following designations:
- pT2 - Tumors showing vascular invasion automatically are classified as pT2, regardless of tumor size and degree of invasiveness; invasion of the tunica vaginalis of itself is also classified as pT2, but solitary involvement of tunica albuginea is not
- pT3 - If the tumor invades the spermatic cord, it is considered pT3
- pT4 - If the tumor goes beyond the spermatic cord to invade the scrotum, it is classified as pT4
- pN1 - Involvement of fewer than 5 lymph nodes by a tumor measuring less than 2cm is classified as pN1
- pN2 - Involvement of more than 5 nodes or any involvement by a tumor measuring 2-5cm is classified as pN2
- pN3 - A lymph node mass measuring greater than 5 cm is classified as pN3
- pM - The category pM is subdivided into M1a (metastasis to nonregional lymph nodes or pulmonary metastasis) and M1b (distant metastasis to sites other than nonregional lymph nodes and lungs)
In addition to classification in accordance with the TNM stage, the classification of testicular tumors incorporates serum marker levels—specifically, lactase dehydrogenase (LDH), human chorionic gonadotropin (hCG), and AFP levels.
Because of clinical implications and the fact that most cases of embryonal carcinoma represent mixed germ cell tumors rather than pure forms, it is the role of pathologist to describe and quantify the various elements of the tumor. It is a known that metastatic disease distinct from the primary tumor may occur years after the initial diagnosis. [25]
Relapse occurs in about 20% of patients for whom embryonal carcinoma is identified in the orchiectomy specimen. Ipsilateral retroperitoneal lymph node dissection may be warranted in patients with a higher percentage of embryonal carcinoma (in some centers, a 40% cutoff is advocated).
Other factors that are associated with occult lymph node metastasis at the time of orchiectomy are an absence of a yolk sac component; the presence of pure embryonal carcinoma; embryonal carcinoma in the absence of teratoma; the presence of choriocarcinoma; proliferating makers (eg, Ki-67); a high level of p53 positivity in embryonal carcinoma cells; elevations in AFP level; and aneuploidy in tumor cells. Spontaneous regression of metastatic embryonal carcinoma has been rarely reported. [26]
Because embryonal carcinomas are aggressive tumors, the percentage of this component in mixed germ cell neoplasms is predictive of tumor progression and metastasis. [27, 28] Embryonal carcinoma has the highest rate of lymphovascular invasion and extension into paratesticular tissue. [29] Clinical stage is, however, the foremost determinant of prognosis in embryonal carcinomas and in other germ cell tumors. [30]
Because retroperitoneal lymph node dissection is capable of eradicating resectable disease in the majority of patients with stage N1-N2 tumors, determination of the amount of embryonal carcinoma in a given tumor may influence the clinical decision to explore the retroperitoneal space. The role of the pathologist is to adequately quantify the percentages of each tumor in the specimen.